Female Sexual Dysfunction

Female sexual dysfunction encompasses a number of conditions that are characterized by one of the following symptoms: loss of sexual desire, impaired arousal, inability to achieve orgasm, or sexual pain. A diagnosis of female sexual dysfunction is made when symptoms are sufficient to result in personal distress. The adverse effect of female sexual dysfunction on the quality of life of affected women can extend into interpersonal relationships and the work-place. In North American culture, female sexual dysfunction is prevalent but often neglected in the health care nesting because women are unlikely to discuss it with their health care providers unless asked. Talking about sexual function with patients may elicit anxiety in the physician and patient. Obstacles to discussing sexual health include a lack of adequate training and confidence in the topic, few perceived treatment options, inadequate clinical time to obtain a sexual history, patients' reluctance to initiate the conversation, and the underestimation of the prevalence of sexual dysfunction. The purpose of this document is to describe the basics of this disorder, including the physiology of the normal female sexual response; outline the criteria for diagnosis as listed in the Diagnostic and Statistical Manual of Mental Disorders: DSM-IV-TR, fourth edition, text revision (DSM-IV-TR); highlight current management strategies based on available evidence; and target areas that require more study.
Background
During the 1950s, Kinsey and colleagues published landmark studies of sexual practices in the United States that examined the sexual lives of females. Masters and Johnson subsequently pioneered research efforts that expanded our scientific knowledge of the sexual response. They identified four physiologic stages of the sexual response:
- Excitement
- Plateau
- Orgasm
- Resolution
These stages are basic biologic responses influenced by psychologic, environmental, and physiologic factors. Later, a three-phase model was developed, consisting of
- Desire
- Arousal
- Orgasm
A more complex, nonlinear model of female sexual response also has been proposed that integrates emotional intimacy, sexual stimuli, and relationship satisfaction.
Desire and arousal are difficult to distinguish as distinct entities, and desire does not always precede arousal. For many women, a sexual encounter may begin without any desire initially present. According to the DSM-IV-TR?, sexual dysfunction generally is characterized as any sexual complaint or problem resulting from disorders of desire, arousal, orgasm, or sexual pain that causes marked distress or interpersonal difficulty. Because more than one female sexual dysfunction may exist in the same patient, it is important that the clinician determine which is the primary female sexual dysfunction and how comorbid female sexual dysfunctions evolved over time.
Normal Sexual Response
 Sexual arousal in women results in increased genital blood flow, swelling of the labia and vaginal walls, release of lubricating secretions from the genital tract, and transudation from the subepithelial vasculature. Vulvae blood flow increases from active neurogenic dilation of sinusoidal blood spaces in the corporal tissue of the clitoris, vestibular bulbs, and spongiosal tissue surrounding the urethra. Pelvic nerve stimulation results in clitoral smooth muscle relaxation and arterial smooth muscle dilation. With increasing arousal, clitoral artery inflow increases clitoral intracavernous pressure, which causes tumescence and protrusion of the clitoris. Central neuroendocrine mechanisms that regulate female sexual response are described today as a dynamic process, creating a balance between excitatory and inhibitory factors. Desire is believed to be triggered in the hypothalamus by the activation of the dopamine system. Research suggests that increased activity of the dopamine system occurs early in the sexual response and may propagate to and activate other areas of the brain, including the limbic system. The noradrenergic system is believed to be involved in sexual arousal through the initiation of autonomic sensations of excitement with increased heart rate and increasing blood pressure (both systolic and diastolic). Orgasm is a transient pealc sensation of intense pleasure and can be described as a reflex with rhythmic contractions of the perineal, bulbocavemosus, and pubococcygeus muscles, with a sudden release of endogenous opioids, serotonin, prolactin, and oxytocin. Resolution has been associated with increased brain serotonergic activity and decreased dopamine release.
Sexual arousal in women results in increased genital blood flow, swelling of the labia and vaginal walls, release of lubricating secretions from the genital tract, and transudation from the subepithelial vasculature. Vulvae blood flow increases from active neurogenic dilation of sinusoidal blood spaces in the corporal tissue of the clitoris, vestibular bulbs, and spongiosal tissue surrounding the urethra. Pelvic nerve stimulation results in clitoral smooth muscle relaxation and arterial smooth muscle dilation. With increasing arousal, clitoral artery inflow increases clitoral intracavernous pressure, which causes tumescence and protrusion of the clitoris. Central neuroendocrine mechanisms that regulate female sexual response are described today as a dynamic process, creating a balance between excitatory and inhibitory factors. Desire is believed to be triggered in the hypothalamus by the activation of the dopamine system. Research suggests that increased activity of the dopamine system occurs early in the sexual response and may propagate to and activate other areas of the brain, including the limbic system. The noradrenergic system is believed to be involved in sexual arousal through the initiation of autonomic sensations of excitement with increased heart rate and increasing blood pressure (both systolic and diastolic). Orgasm is a transient pealc sensation of intense pleasure and can be described as a reflex with rhythmic contractions of the perineal, bulbocavemosus, and pubococcygeus muscles, with a sudden release of endogenous opioids, serotonin, prolactin, and oxytocin. Resolution has been associated with increased brain serotonergic activity and decreased dopamine release.
Types of Sexual Dysfunction
Female sexual dysfunction conditions can be categorized as sexual desire disorders, sexual arousal disorder, orgasmic disorder, or sexual pain disorders.
Sexual Desire Disorders
Hypoactive sexual desire disorder and sexual aversion disorder comprise the sexual desire disorders. According to the DSM-IV-TR, hypoactive sexual desire disorder is defined as a persistent or recurrent deficiency or absence of sexual desire or receptivity to sexual activity that causes marked distress or interpersonal difficulty. Sexual aversion disorder is defined as a persistent or recurrent aversive response to genital contact with a sexual partner that causes distress or interpersonal difficulty.
Hypoactive sexual desire disorder is the most common female sexual dysfunction, with an estimated prevalence rate ranging between 5.4% and 13.6% (See Figure below). One study reported an 83% prevalence of hypoactive sexual desire disorder based on a representative sample of almost 2,000 U.S. women aged 30-70 years.
Hypoactive sexual desire disorder reaches a peak in women aged 40-60 years (see below Figure) and in individuals that have undergone surgical menopause. In this age group, the disorder can be linked to situational circumstances, such as chronic disease, depression, or medication use, but more often is diagnosed as an isolated event. Atrophic vaginitis and pelvic floor surgery can lead to dyspareunia and sexual aversion and lost sexual desire. Women with endocrine problems and adrenal insufficiency also frequently experience hypoactive sexual desire disorder.
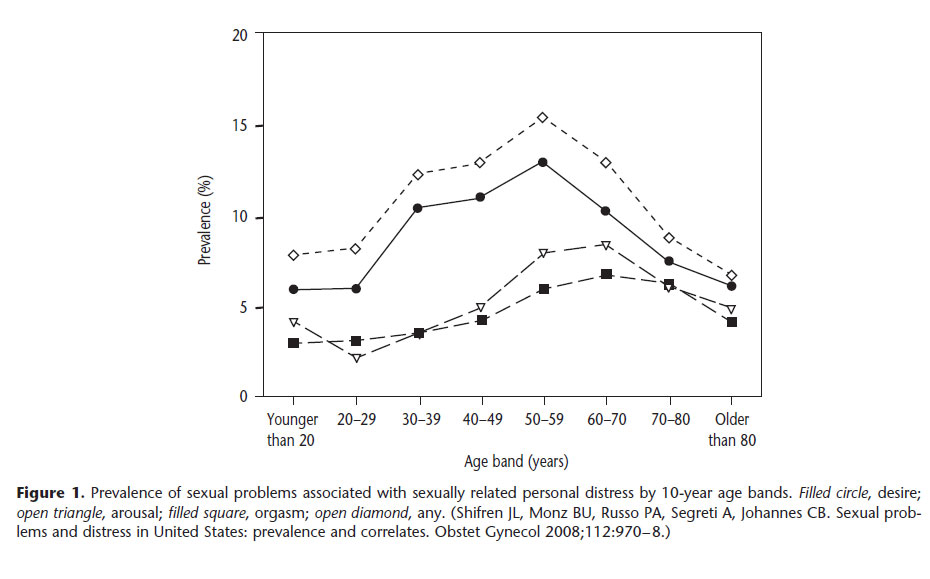
In young women, hypoactive sexual desire disorder frequently is associated with situational circumstances, such as dysfunctional interpersonal relationships, chronic disease, depression, use of certain medications, gynecologic disorders, or other mitigating factors. Use of antidepressants, particularly selective serotonin reuptake inhibitors (SSRIs), oral contraceptives, and corticosteroids can be associated with hypoactive sexual desire disorder.
The prevalence of sexual aversion disorder is not well established. Painful or traumatic life events may give rise to sexual aversion. Because many women with this disorder avoid sexual contact, the disorder may remain undiagnosed unless it surfaces as part of a dysfunctional relationship. Treatment consists of psychotherapy and antidepressants for patients who have associated anxiety.
Female Sexual Arousal Disorder
Female sexual arousal disorder refers to an inability to complete sexual activity with adequate lubrication that causes marked distress or interpersonal difficulty.
The results from a survey of a national research panel representative of U.S. women indicate that 5% of these women have significant difficulty with sexual arousal. Because female sexual arousal disorder frequently is linked to a gynecologic or chronic medical condition or the use of certain medications, it typically resolves when the inciting disorder is successfully treated or the medication is adjusted. Medications, particularly SSRIs, are commonly associated with female sexual arousal disorder. Female sexual arousal disorder also may be associated with atrophic vaginitis after spontaneous menopause or oophorectomy, which causes pain with vaginal penetration and difficulties in lubrication that impair sexual arousal.
Female Orgasmic Disorder
 The DSM-IV-TR defines female orgasmic disorder as a persistent or recurrent delay in or absence of orgasm after a normal excitement phase, which causes marked distress or interpersonal difficulty. Female orgasmic disorder has a reported prevalence of 3.4-5.8 %.
The DSM-IV-TR defines female orgasmic disorder as a persistent or recurrent delay in or absence of orgasm after a normal excitement phase, which causes marked distress or interpersonal difficulty. Female orgasmic disorder has a reported prevalence of 3.4-5.8 %.
Primary orgasmic disorder is defined as never having the ability to achieve orgasm. Women with primary orgasmic disorder usually have normal levels of sexual desire but are unable to achieve orgasm. Primary orgasmic disorder often is associated with a history of trauma or abuse or can have genetic origins, but it may have no explanation. It usually does not resolve on its own. In primary orgasmic disorder associated with abuse, psychotherapy and couples counseling may be helpful. After counseling, masturbation is an effective way for the woman who has never achieved orgasm to experience her first climax. There is no effective therapy for unexplained primary orgasmic disorder in which the patient has never achieved orgasm even through masturbation.
Secondary orgasmic disorder generally is the result of another sexual dysfunction. Secondary orgasmic disorder frequently is linked with hypoactive sexual desire disorder, having the same situational and psychosocial causes. It can be associated with pelvic surgery and medications, such as antidepressants. In women with orgasmic dysfunction, SSRIs are a commonly recognized cause. A number of psychosocial factors, including age, social class, personality, and relationship status have been commonly related to orgasmic ability. Religious and cultural beliefs have been found to be negatively correlated with orgasmic ability, a finding that is believed to be due to individuals' feelings of excessive guilt about participating in sexual activity.
Treatment of the primary dysfunction frequently leads to restoration of the ability to achieve orgasm. Women are taught to be comfortable with their bodies as well as their own sexuality by altering negative attitudes and decreasing anxiety. Adjunctive education on self-pleasuring techniques generally is helpful. Behavioral treatments include masturbation instruction, communication exercises, sensate focus exercises, and systematic desensitization.
Sexual Pain Disorders
Dyspareunia and vaginismus are two subcategories of sexual pain disorders. According to the DSM-IV-TR, dyspareunia is defined in as recurrent or persistent genital pain associated with sexual intercourse that is not caused exclusively by lack of lubrication or by vaginismus and causes marked distress or interpersonal difficulty. The DSM-IV-TR defines vaginismus as recurrent or persistent involuntary spasm of the musculature of the outer third of the vagina that interferes with sexual inter-course, causing marked distress or interpersonal difficulty.
Dyspareunia is a common sexual problem, particularly in postmenopausal women in which prevalence ranges from 8% to 22%. Recent perspectives suggest that dyspareunia may be characterized as a pain disorder that interferes with sexuality rather than as a sexual disorder characterized by pain. Therefore, dyspareunia is believed to be a specific pain disorder with interdependent psychologic and biologic contributions and context-dependent etiologies. Pain on vaginal entry typically is reflective of provoked vestibulodynia, inadequate lubrication, or vaginismus. Physical examination will reproduce the pain when the vulva or vagina is touched with a cotton swab or when a finger is inserted into the vagina. Palpation of the walls of the vagina, uterus, and urethral structures can help identify physiologic contributions. Identification of the initiating and maintaining factors is fundamental to the diagnostic process. Loss of desire and arousal disorders associated with dyspareunia may contribute to the worsening of pain over time because the lack of genital arousal paired with sexual activity often results in physical discomfort. The differential diagnosis is broad (see below table).
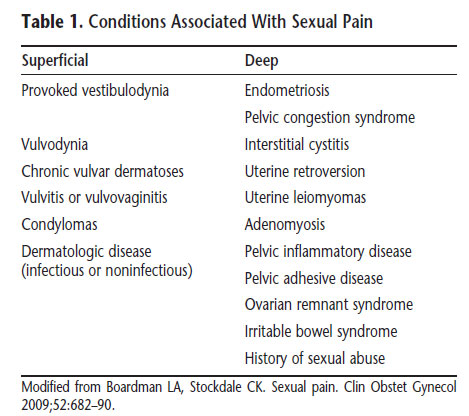
Vaginismus is a relatively uncommon problem with prevalence rates ranging from 1% to 6%. In some women, vaginismus occurs because pain is anticipated. For some women, vaginismus is limited to sexual activity, whereas in others it is related only to fear of pelvic examination. Some women enjoy sexual activity and achieve orgasm, but still have vaginismus; they cannot consummate intercourse because vaginal penetration is not possible. Vaginismus frequently is linked to hypoactive sexual desire disorder and sexual aversion. These disorders often have the same situational and psychosocial causes and resolve in response to treatment of those conditions. In other cases, vaginismus is linked to gynecologic disorders, chronic medical conditions, or the use of certain medications, and it resolves with treatment or medication adjustment.
The most effective treatment is a combination of cognitive and behavioral psychotherapy, typically refer-red to as systematic desensitization. Women are taught deep muscle relaxation techniques, which they then use during exercises in which they are instructed to very gradually insert objects (usually dilators) of increasing diameter into the vagina. The goal is to desensitize a woman to her fear that vaginal penetration will be painful and to enable her to gain a sense of control over a sexual encounter or a pelvic examination, so that vaginal muscle contractions no longer occur as an automatic defense to vaginal penetration. If treatment is not progressing, referral for pelvic floor physical therapy often is helpful.
Clinical Considerations and Recommendations
What is the initial approach to a patient who presents with a possible sexual dysfunction?
 A physician who is comfortable with the topic, knows and has seen the patient before, is caring and compassionate, and seems concerned about sexual wellness is one with whom patients will feel comfortable discussing sexual concerns. The initial approach begins with obtaining a sexual history during the review of symptoms. A very brief net of questions can suffice or the patient can complete a screening questionnaire. The Brief Sexual Symptom checklist for women was developed by the International Consultation in Sexual Medicine m a primary screening tool and may be helpful (see Box on the side). A questionnaire provides an opportunity to let the patient know that discussing sexual health is important and appropriate. Taking a thorough sexual history includes recording the patient's medical, surgical, social, and psychiatric history. Information about the use of prescription and over-the-counter medications should be elicited and a complete gynecologic evaluation performed, targeting areas that were uncovered in the sexual function history. After initial evaluation, treatment can be initiated or, depending on the comfort level and training of the physician, a referral can be made to a trained specialist, such as a marriage counselor or sex therapist.
A physician who is comfortable with the topic, knows and has seen the patient before, is caring and compassionate, and seems concerned about sexual wellness is one with whom patients will feel comfortable discussing sexual concerns. The initial approach begins with obtaining a sexual history during the review of symptoms. A very brief net of questions can suffice or the patient can complete a screening questionnaire. The Brief Sexual Symptom checklist for women was developed by the International Consultation in Sexual Medicine m a primary screening tool and may be helpful (see Box on the side). A questionnaire provides an opportunity to let the patient know that discussing sexual health is important and appropriate. Taking a thorough sexual history includes recording the patient's medical, surgical, social, and psychiatric history. Information about the use of prescription and over-the-counter medications should be elicited and a complete gynecologic evaluation performed, targeting areas that were uncovered in the sexual function history. After initial evaluation, treatment can be initiated or, depending on the comfort level and training of the physician, a referral can be made to a trained specialist, such as a marriage counselor or sex therapist.
Which medications are associated with female sexual dysfunction?
Numerous medications, both prescription and over-the-counter, have been associated with sexual dysfunction. Psychotropic medications, antihypertensives, histamine blockers, and hormonal medications also have been implicated.
The most common medications linked to sexual dysfunction are the SSRIs. The most frequently reported problems are orgasmic dysfunction, decreased sexual desire, and decreased arousal. Compounding this side effect is the fact that depressed women tend to have sexual dysfunction before treatment begins.
Decreasing the dosage of a medication sometimes may help alleviate some of these problems. Switching to another antidepressant may alleviate symptoms, but other antidepressant classes also have been associated with sexual dysfunction. Although a structured treatment interruption may be helpful in some patients, it is not an option in some other patients because of under-lying psychiatric concerns. Consultation with a health care provider with expertise in psychiatric medications who can assist in distinguishing baseline female sexual dysfunction from dysfunction resulting from treatment of depression may be helpful. A medication adjustment with long-term follow-up may be important for improved sexual functioning.
What is the effect of hysterectomy on sexual function? What is the effect of supracervical hysterectomy on postoperative sexual function compared with hysterectomy with removal of the cervix?
The main indications for hysterectomy in the United States are uterine leiomyomas, menstrual disorders, uterine prolapse, and endometriosis—all of which can lead to a decreased quality of life and sexual dysfunction. Aside from the risks inherent in the surgery itself, anxiety about sexual function after surgery is high.
Postoperative dyspareunia, resulting from shortening vaginal length at the time of abdominal hysterectomy; postoperative scarring of the vagina, resulting from vaginal dryness; and the possibility that orgasm would not be as strong or pleasurable without a uterus have been proposed as reasons for decreased sexual function postoperatively. However, there are many prospective studies that document improved dyspareunia rates after hysterectomy, regardless of operative route.
In general, prospective studies constructed to address the effect of hysterectomy on postoperative sexual function have failed to show a difference in total versus subtotal hysterectomy. One study of sexual satisfaction reported similar rates preoperatively and 1 year postoperatively by women, irrespective of type of hysterectomy performed. A second study comparing supracervical hysterectomy and total abdominal hysterectomy reported similar findings in the frequency of intercourse, frequency of orgasm, and rating of sexual relationship with a partner measured preoperatively and postoperatively for women in both groups. In a third study, there were no differences between the supracervical hysterectomy and the total abdominal hysterectomy groups in sexual functioning and measurement of health-related quality of life, including sexual desire, orgasm frequency and quality, and body image, measured 2 years after surgery.
What is the role of estrogen therapy on sexual function?
The results of recently conducted hormonal supplementation studies have prompted clinicians to re-evaluate universal estrogen use in postmenopausal women, in oral or topical form. Estrogen affects sexual performance through maintenance of genital tissues and secretions, pelvic muscle tone, and elasticity. The addition of topical estrogen to the vagina can aid lubrication by reducing intercellular space resistance and can improve fluid flow through the epithelium. Vaginal estrogen for the treatment of postmenopausal atrophy results in improved dyspareunia, less vaginal dryness, improved vaginal mucosal maturation indices, and reduced vaginal pH. Genital estrogen effects are best understood by the consequences of their absence. Estradiol secretion is variable during perimenopausal years and decreases to very low levels after menopause. Estrogen withdrawal increases tissue fragility, rates of vaginal and urinary infections, irritation, dryness, urogenital pain, and susceptibility to vaginal tissue trauma. Decreasing estrogen levels induce vulvovaginal atrophy leading to sexual pain and trauma during intercourse.
Oral forms of estrogen may not alleviate vulvovaginal atrophy and topical estrogen may be required. For vulvovaginal atrophy leading to sexual dysfunction, topical estrogen formulations are the most effective. Tablets, gels, creams, and vaginal rings appear to be equally effective, and selection of an estrogen formulation should incorporate patient’s preference. Systemic absorption of vaginal estrogen is limited, but still a concern because serum levels of estrogen in a treated patient are higher than in the nontreated patient. The lowest effective dose should be used for the least amount of time to alleviate symptoms. The duration of treatment has not been determined, but some experts advocate daily treatment for a period of a few weeks, tapering down after this period based on symptoms.
Nonestrogen lubricants also may be useful for those women who cannot or choose not to take estrogen. These water-based or silicone-based lubricants and moisturizers do not address underlying causes of sexual dysfunction, but may be helpful in reducing or alleviating dyspareunia.
In women with orgasmic disorders, what is the evidence for the efficiency of vasoactive medications (i.e., sildenafil)?
Sildenafil citrate is believed to increase pelvic blood flow to the clitoris and vagina similar to that in men who are being treated for erectile dysfunction. In randomized clinical trials of women being treated for sexual arousal disorder, the results have been contradictory. Clinical trials of sildenafil were conducted to address the lack of vasocongestion. Vaginal engorgement in the presence of sexual stimuli was demonstrated with the use of sildenafil, but subjective experience of arousal was not reliably achieved. One study of 98 women treated for depression with an SSRI compared sildenafil with placebo to improve orgasmic dysfunction and noted an improvement in Clinical Global Impression Sexual function scores in the sildenafil group. However, additional research is needed before a recommendation can be made for the use of sildenafil for the treatment of female sexual dysfunction.
What is the evidence for the safety and efficacy of devices to treat arousal disorders?
One battery-powered device intended for clitoral therapy has received approval from the US. Food and Drug Administration (FDA). This device is applied directly over the clitoris to create a vacuum to increase blood flow and engorgement. Several small pilot studies have evaluated its efficacy in improving orgasm, vaginal lubrication, genital sensation, and sexual satisfaction. This device may be best suited for women with arousal and orgasm difficulties, and no adverse outcomes from use of the device have been noted.
What formulations and what routes of administration are preferred for androgen therapy in the treatment of hypoactive sexual desire disorder?
 Androgen levels continue to decrease in reproductive-aged women until menopause, at which point no further decrease is observed. Numerous studies have demonstrated that sexual desire and sexual activity increase with androgen supplementation, but there also are as many that are equivocal in this regard. There are little long—term prospective data on the use of androgen therapy for female sexual dysfunction. Testosterone, the most commonly used androgen replacement treatment, does not have FDA approval for the treatment of hypoactive sexual desire disorder. Transdermal testosterone has been shown to be effective for the short-term treatment of hypoactive sexual desire disorder in women, with little evidence to support long-term use (longer than 6 months). There is no proven clinical utility to monitoring androgen levels before or during treatment.
Androgen levels continue to decrease in reproductive-aged women until menopause, at which point no further decrease is observed. Numerous studies have demonstrated that sexual desire and sexual activity increase with androgen supplementation, but there also are as many that are equivocal in this regard. There are little long—term prospective data on the use of androgen therapy for female sexual dysfunction. Testosterone, the most commonly used androgen replacement treatment, does not have FDA approval for the treatment of hypoactive sexual desire disorder. Transdermal testosterone has been shown to be effective for the short-term treatment of hypoactive sexual desire disorder in women, with little evidence to support long-term use (longer than 6 months). There is no proven clinical utility to monitoring androgen levels before or during treatment.
Transdermal testosterone delivered by a matrix patch is the most extensively studied of the systems. However, matrix patches are not approved by the FDA and, therefore, are not available in the United States for the treatment of hypoactive sexual desire disorder. There are numerous randomized blinded clinical trials of the use of this matrix patch by nearly 3,000 postmenopausal women (in whom menopause occurred naturally or was surgically induced) with hypoactive sexual desire disorder. All trials have demonstrated dose-related, significant increases in sexual desire with testosterone patches versus placebo when the dose was maintained at 300 micrograms per day or greater. All trials used a matrix patch that delivered testosterone at various doses (150 micrograms per day, 300 micrograms per day, or 450 micrograms per day), depending on the experimental design. The dosages investigated approximated the lower and higher limits of normal production of testosterone in premenopausal women. At 300 micrograms per day, consistent increases in sexual desire with few adverse effects were seen, but at 150 micrograms per day, the improvements were borderline. Side effects in these first 24-week trials were minimal and not different between groups except for patch site irritation and hirsutism, which was minor.
There are fewer studies of testosterone use in premenopausal women for the treatment of hypoactive sexual desire disorder. In a randomized controlled trial of 31 women (aged 31—45 years) using testosterone cream, those women showed statistically significant increases on validated self-reported sexual function scales at 12 weeks. In a larger trial of 261 women (aged 35—46 years), the use of testosterone spray, in three different doses, was shown to statistically increase sexually satisfying events with the higher two doses compared with placebo at 16 weeks.
Methyltestosterone, micronized testosterone, and dehydroepiandrosterone are available either off label or as customized formulations. However, there are limited, prospective, randomized high-quality clinical trial data on this treatment. Consensus reports from the Endocrine Society and the North American Menopause Society have been cautionary.
What are the risks of androgen therapy, and how should patients be monitored?
The main risks associated with androgen replacement therapy in women are hirsutism, acne, virilization, and cardiovascular (CV) complications. In addition, a possible association with breast cancer has been reported.
Hirsutism
In studies in which women were administered testosterone for arousal disorders or vasocongestion difficulties, hirsutism, or unwanted hair growth, affected 3—20% of those treated. In a large prospective trial of transdermal testosterone (placebo versus 150 micrograms versus 300 micrograms) for hypoactive sexual desire disorder, hair growth increased with increasing testosterone doses, although free testosterone levels had little correlation with the degree of hirsutism, and the group that experienced increased hair growth was not more likely to discontinue therapy.
Acne
Acne has been noted in less than 10% of patients who are treated with testosterone. In trials that compared testosterone plus estrogen with estrogen alone, there was no difference noted in acne prevalence.
Virilization
Virilization is uncommon and is mainly seen in supraphysiologic doses (97), with few women developing clitoromegaly, deepening of the voice, increase in muscle mass, and temporal balding in the dosages used for hypoactive sexual desire disorder treatment. When virilization is noted in patients receiving smaller doses, the condition usually is reported as mild.
Cardiovascular Risk
There are little prospective long-term data regarding adverse CV effects in women receiving testosterone for hypoactive sexual desire disorder. However, methyltestosterone combined with esterified estrogens has been associated with significant decreases in high-density lipoprotein (HDL) cholesterol and increased total cholesterol-to-HDL ratio but a significant decrease in triglycerides (98). There are little prospective long-term data regarding adverse CV effects in women receiving testosterone for hypoactive sexual desire disorder. In female-to-male transsexuals taking supraphysiologic doses of testosterone (160 mg/d of oral testosterone undecanoate), the risk of adverse CV effects (myocardial infarction, hypertension, and CV death) were no more than expected in the general male population.
Monitoring, consisting of serum lipid measurements and liver function tests in those women who are to begin androgen replacement seems reasonable. More long-term data in women receiving testosterone are needed to answer these questions.
Breast Cancer
Breast cancer has been reported in a trial of the treatment of hypoactive sexual desire disorder, but it is unclear whether this was due to chance or if there is a causal relationship. The Women’s Health Initiative observational trial reported a nonsignificant increase in the number of invasive breast cancer cases in those women taking testosterone and estrogen versus those in the control group (adjusted hazard ratio, 1.42; 95% confidence interval, 0.95-2.11).